Background:
Resistance to venetoclax, a BCL2 inhibitor, is mediated by co-expression of other BCL2 proteins including BCLXL and MCL1. In vitro data show that DNA damage reduces the level of MCL1, a known mediator of venetoclax resistance. The monoclonal antibody radioconjugate, lintuzumab-Ac225, is a highly cytotoxic alpha-radiation emitter that selectively targets CD33, a cell surface antigen expressed on the majority of AML cells. Clinical studies have shown that the high-energy alpha-particle emissions from lintuzumab-Ac225 elicit single and double-stranded DNA breaks in targeted tumor cells. In venetoclax-resistant cell lines in vitro, lintuzumab-Ac225 promotes MCL1 degradation through DNA damage resulting in increased cell sensitivity to venetoclax. Similarly, mice xenografted with venetoclax-resistant AML tumor lines demonstrated tumor regression and increased survival when treated with venetoclax and lintuzumab-Ac225. The aims of this phase I/II study are to assess the safety, tolerability and efficacy of lintuzumab-Ac225 in combination with venetoclax in R/R AML.
Study Design:
The Phase I portion of the study used a 3+3 dose-escalation design to determine the maximum tolerated dose (MTD) of lintuzumab-Ac225 combined with venetoclax. The dose levels for lintuzumab-Ac225 were 0.5, 0.75, 1.0 µCi/kg, 1.5 µCi/kg, and 2.0 µCi/kg (expansion cohort). The Phase II portion of the study was designed to enroll up to an additional 20 patients at the recommended phase II dose (RP2D) with venetoclax to determine the best overall response (CR+CRh+CRi) up to 6 months after starting treatment.
Eligible patients included R/R AML patients aged 18 years and older with adequate organ function, ECOG performance status 0-2, and more than 25% CD33 positive leukemic blasts by flow cytometry. Patients with antecedent myelodysplastic syndromes, myeloproliferative neoplasms, or therapy-related AML were eligible. Venetoclax was given at a dose of 400 mg daily on days 1 to 21 (cohorts 1-3) or days 2-22 (cohort 4). Lintuzumab-Ac225 was administered as a single dose of each cycle on day 5 (cohorts 1-3) or day 1 (cohort 4).
Results:
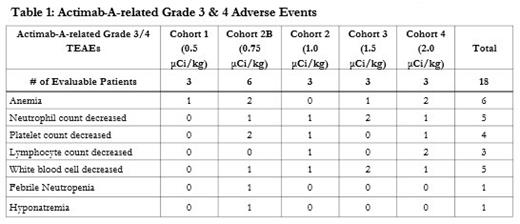
In total, eighteen R/R AML patients received lintuzumab-Ac225 at 0.5 µCi/kg (n=3), 0.75 µCi/kg (n=6), 1.0 µCi/kg (n=3), 1.5 µCi/kg (n=3) and 2.0 µCi/kg (n=3) with venetoclax in the phase I dose escalation portion. The median age was 73 years (range 46-87); 7/18 (39%) had refractory AML and 9/18 (50%) had ELN unfavorable risk. In the dose escalation phase, one DLT with grade 3 prolonged thrombocytopenia occurred in 1 of 6 patients received lintuzumab-Ac225 at 0.75 µCi/kg plus venetoclax. No DLTs were observed at 1.0 µCi/kg (Cohort 2), 1.5 µCi/kg (Cohort 3) and 2.0 µCi/kg (Cohort 4) of lintuzumab-Ac225 plus venetoclax. Grade 3 or higher adverse events related to lintuzumab-Ac225 treatment included hematologic AEs and one count of non-hematologic event (hyponatremia) (Table 1). In cohort 4 at 2.0 µCi/kg of lintuzumab-Ac225 with the dosing schedule modification, reduction of BM blasts was observed in 2 of 3 patients. One patient achieved MLFS with 93% reduction of BM blasts.
Conclusion:
The combination of lintuzumab-Ac225 and venetoclax had a manageable safety profile and no early mortality at day 30. The MTD was not reached and no significant toxicities have been reported during the follow-up period. Modified dosing schedule in Cohort 4 of the combination demonstrated improved anti-leukemic effects. The data observed supports the development of further trials to evaluate the safety and anti-leukemic efficacy of lintuzumab-Ac225 in combination with venetoclax-based treatment in the R/R AML population.
Disclosures
Roboz:Bluebird bio: Consultancy; Actinium: Consultancy; GSK: Consultancy; Janssen: Consultancy, Research Funding; AZ: Consultancy; BMS: Consultancy; Blueprint: Consultancy; Agios: Consultancy; Amgen: Consultancy; Astellas: Consultancy; Jasper: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Mesoblast: Consultancy; AbbVie: Consultancy; Jazz: Consultancy; MEI: Consultancy; Syndax: Consultancy; Takeda: Consultancy. Orozco:Actinium Pharmaceuticals: Other: Site PI for clinical trials sponsored by Actinium, Research Funding. Desai:Actinium Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Chen:Actinium Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Schiller:Sangamo Bioscience: Research Funding; Incyte: Consultancy, Research Funding, Speakers Bureau; Geron: Research Funding; Mateon Therapeutics: Research Funding; Onconova Therapeutics: Research Funding; Celator: Research Funding; Pfizer: Research Funding; Kronos Bio: Research Funding; Actuate Therapeutics: Research Funding; AbbVie: Consultancy, Research Funding, Speakers Bureau; Sanofi: Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding; Actinium Pharmaceuticals: Research Funding; Sellas Life Sciences: Research Funding; Stemline Therapeutics: Research Funding; Agios: Consultancy; ElevateBio: Research Funding; Celgene: Consultancy, Research Funding; Arog: Research Funding; Takeda: Research Funding; Karyopharm Therapeutics: Research Funding, Speakers Bureau; Ono Pharmaceutical: Consultancy; Astellas Pharma: Consultancy, Research Funding, Speakers Bureau; Deciphera: Research Funding; Constellation Pharmaceuticals: Research Funding; Syros Pharmaceuticals: Research Funding; Ono Pharmaceutical: Research Funding; Trovagene: Research Funding; Tolero Pharmaceuticals: Research Funding; Samus Therapeutics: Research Funding; REGiMMUNE: Research Funding; Precog: Research Funding; Genentech/Roche: Research Funding; Gamida Cell: Research Funding; Fujifilm: Research Funding; FORMA Therapeutics: Research Funding; Delta-Fly Pharma: Research Funding; Stemline Therapeutics: Speakers Bureau; Kite: Research Funding, Speakers Bureau; Agios: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; AVM Biotechnology: Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Amgen: Current equity holder in publicly-traded company, Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal